Answer: 10 micrograms per liter of urine is below the legal safe limit and the dentist is not at risk for mercury poisoning.
Step-by-step explanation:
To calculate the number of moles, we use the formula:

We are given:
Given mass of mercury =
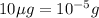 (Conversion factor:
(Conversion factor:
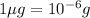 )
)
Molar mass of mercury = 200.6 g/mol
Putting values in above equation, we get:
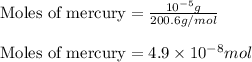
According to mole concept:
1 mole of an element contains
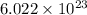 number of atoms
number of atoms
So,
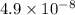 moles of mercury will contain =
moles of mercury will contain =
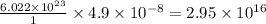 number of atoms.
number of atoms.
We are given:
Legal safe limit for mercury in urine =
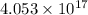 atoms
atoms
Calculated amount of mercury in urine =
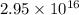 atoms
atoms
As, the calculated amount of mercury in urine is less than the legal safe limit of mercury in urine. So, the average dentist is not at risk for mercury poisoning.
Hence, 10 micrograms per liter of urine is below the legal safe limit and the dentist is not at risk for mercury poisoning.