Answer:
b. There are less than 40 protons in this atom.
Step-by-step explanation:
Hello.
In this case, given the information we have available in the periodic table, we can infer that the number of both protons and electrons of neutral atoms is equal to the atomic number, not the atomic mass which is actually given. Nevertheless, the number neutrons are computed by knowing the atomic mass and the atomic number via:
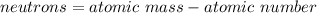
It means that the atomic mass is greater than the atomic number or the amount of both protons and electrons. In such a way, we can infer that the answer is b. There are less than 40 protons in this atom since the number of neutrons must be positive.
Best regards.