Answer:
(a) 10 quantities of protein
(b) 2 quantities of protein
Step-by-step explanation:
Given
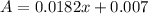
Solving (a):
Solve for x when A = 0.207
Substitute 0.207 for A
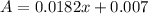
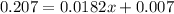
Solve for 0.0182x
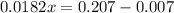
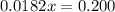
Solve for x
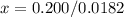
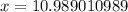
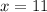 (Approximated)
(Approximated)
Solving (b):
Solve for x when A = 0.050
Substitute 0.050 for A
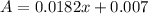
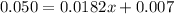
Solve for 0.0182x
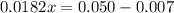
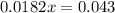
Solve for x
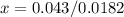
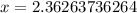
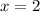 (Approximated)
(Approximated)