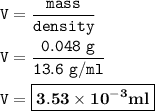Answer : 3.53x10⁻³ ml
Further explanation
Density : the ratio between mass and volume
Can be formulated :
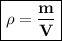
ρ = density, kg/m³ or g/ml
m = mass, kg or g
V = volume = m³ or ml
While mol : dividing mass with molar mass(MW=molecular weight)
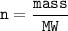
n= mol
mass = grams
MW = molecular weight ,g/mol
Known
moles : 0.00024 moles of Mercury(Hg)
MW of Hg = 200,59 g/mol
so the mass of Mercury :

Because the density of Mercury is 13.6 g/mL, then the volume :