Answer:
85.9%
Step-by-step explanation:
There are two reactions to take into consideration for this problem:
The first one is the reaction of limestone with HCl:
1) CaCO₃ + 2HCl → CaCl₂ + CO₂ + H₂O
While the second one is the titration of HCL with NaOH:
2) HCl + NaOH → H₂O + NaCl
So first we calculate the number of HCl moles added:
- 0.050 L * 0.1035 M = 5.175x10⁻³ moles HCl
A number of those 5.175x10⁻³ moles reacted with limestone while the remaining excess reacted with NaOH.
The number of NaOH moles used in the titration is:
- 0.01662 L * 0.1018 M = 1.692x10⁻³ mol NaOH
We know from equation 2) that the moles of NaOH are equal to the moles of HCl in the titration process.
That means that the number of HCl moles that reacted with CaCO₃ in the limestone sample is:
- 5.175x10⁻³ moles HCl - 1.692x10⁻³ mol HCl = 3.483x10⁻³ mol HCl
Now we use that number of HCl in equation 1) to calculate the number of CaCO₃ moles, which we then convert to grams using its molecular weight:
- 3.483x10⁻³ mol HCl *
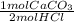 * 100 g/mol = 0.1741 g CaCO₃.
* 100 g/mol = 0.1741 g CaCO₃.
Finally we calculate the CaCO₃ percentage in the sample:
- 0.1741 g / 0.2027 g * 100% = 85.9%