Given parameters:
Mass of sucrose = 5g
Density of sucrose = 1.12g/mL
Percentage of sucrose per liter of cane juice = 12%
Unknown:
Volume of cane juice needed = ?
We need to establish the volume - density relationship. Density is the mass of a substance per unit volume.
Mathematically;
Density =

Now solve for the volume of sucrose;
1.12g/mL =
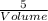
Volume =
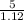 = 4.46mL = 4.46 x 10⁻³L since 1000mL = 1L
= 4.46mL = 4.46 x 10⁻³L since 1000mL = 1L
Since 12% of 1 liter of cane juice is sucrose;
12% of x liter of cane juice = 4.46 x 10⁻³L
Volume of cane juice = 4.46 x 10⁻³ x
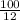 = 0.037L
= 0.037L
Volume of cane juice is 0.037L