Answer:
4 moles
Step-by-step explanation:
A mole is equal to 6.02214076 × 1023 of any chemical unit (atoms, molecules, ions)
To find number of moles in 89 litres of water vapor use the following formula:
1 mole = 22.4 L
That is 1 L =
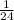 mole
mole
Volume of water vapor = 89 litres
Therefore,
Number of moles in 89 litres =
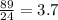 ≈ 4 moles
≈ 4 moles