Answer:
n=0.852 moles
Step-by-step explanation:
Given mass is, m = 19.6 g
The molar mass of sodium is, M = 22.99 u
We need to find the no of moles in 19.6 of Sodium. We know that, no of moles is equal to given mass divided by molar mass.
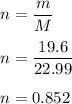
So, there are 0.852 moles in 19.6 g of Sodium.