Answer:
- 0.0062AU
- 0.00082.
- 106.7% error
Step-by-step explanation:
a.) Average or mean value of readings,
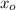 = (0.0044 + 0.0079 + 0.0062) / 3
= (0.0044 + 0.0079 + 0.0062) / 3
=
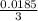 = 0.0062 AU
= 0.0062 AU
b.) The accuracy of the readings is determined by taking determining the standard deviation (SD) of the readings:
SD = ∑
 /n
/n
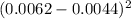 = 0.0000032
= 0.0000032
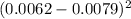 = 0.00000289
= 0.00000289
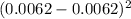 = 0
= 0
SD =
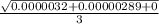 = 0.00082.
= 0.00082.
c.) The % accuracy is the distance between the average value and true value
=
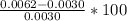 = 106.7% error.
= 106.7% error.