Given parameters;
Mass of gold colored metal beads = 425g
Volume of water displaced by beads = 48.0cm³
Unknown;
Identity of the metal = ?
Given densities;
Gold: 19.3 g/mL
Copper: 8.86 g/mL
Bronze: 9.87 g/mL
Density is an intensive property of any substance. This implies that we can use the density of any substance to identify it.
Density can be defined as the mass per unit volume of a substance. Every substance has a unique mass per volume.
Mathematically;
Density =

where mass is in kg or g
volume is in m³ or cm³
To find the density, we must know the mass and volume.
In this problem, the volume of the gold metal beads is the same as the volume of water displaced. This is a way to measure volume of solids.
Since the volume is given in cm³, and we are comparing with choices that have units in g/mL, we simply convert the volume in cm³ to mL
1cm³ = 1mL³
So therefore, volume of gold colored metal is 48mL
Now input the parameters given and solve for the density;
Density =
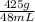 = 8.85g/mL
= 8.85g/mL
From the given densities, we clearly see that copper is the metal since they both of similar densities.