Answer:
C=O
Step-by-step explanation:
The nuclear magnetic resonance (NMR) spectroscopy is a method that employs the technique to observe the local magnetic fields are atoms. This analytical technique exploits sufficient differences in the frequency of nuclear magnetic resonance in protons and atoms within organic compounds that depend on their neighboring atoms.
Amongst all the given options, the carbonyl is the right option because the carbon is doubly bonded to the oxygen and the hybridization of this double bond is sp² hybridized, as a result, the electron density at the carbon possess a lower amount in order to have more magnetic field.
The increasing order of these chemical shifts are as follows:
C - X <
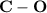 ≅
≅
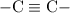 < -C≡N < C=O
< -C≡N < C=O