Answer:
Titanium.
Step-by-step explanation:
Hello,
In this case, since chromium has 24 electrons (atomic number) in order to represent the electron configuration of Cr⁺² which means that it has lost two electrons in order to get positively charged, we must perform an electron configuration until 22 (24-2) as follows:
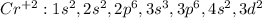
In such a way, adding the electrons per sublevel and shell we count 22, which matches with the number of electrons of titanium and therefore with its very same electron configuration.
Best regards.