Answer:
The answer is below
Step-by-step explanation:
Charles law states that the volume and temperature of a fixed amount of a gas is directly proportional to each other provided that pressure is held constant.
Boyle's law states that the volume and pressure of a fixed amount of a gas are inversely proportional to each other provided that temperature is held constant.
a) Constant property:
In Charles law, pressure is kept constant while in Boyle's law, temperature is held constant.
b) Varying properties:
In Charles law, volume and temperature are varying while in Boyle's law, pressure and volume are varying.
c) Type of variance:
In Charles law we have a direct variance while in Boyle's law we have an indirect variance.
d) Charles law is given as:
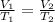
Boyle's law is given as:
