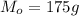Answer:
The value is
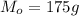
Step-by-step explanation:
Generally the reaction between oxygen and magnesium is
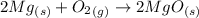
Generally from the law of mass conservation in a chemical reaction we have that
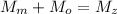
Here
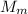 is the mass of magnesium
is the mass of magnesium
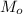 is the mass of oxygen
is the mass of oxygen
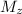 is the mass of magnesium oxide
is the mass of magnesium oxide
So
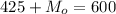
=>
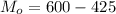
=>