Answer:
E. None of these
Step-by-step explanation:
We know, By GAS laws,
PV = NRT, where p- pressure, v- volume, n- number of moles, R- gas constant ,and T- temperature
Now, In the question, the number of moles remains the same as the gas is the same. so n is constant so we can compare n before and after a temperature change.
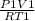 =
=
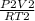
where P1= 1 atm, P2 = 10 atm, V1= 20 mL, T1= 10°C and T2= 100°C
We don't have to worry about the standard units as they are present equally on both the sides and get cut, same goes for R( gas constant)
So putting values, we get
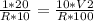
Cutting, R on both sides and moving contents to the right so that only V2 is left on the left.
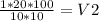
∴ V2 =
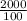
∴ V2 = 20mL