Answer:
Explanat
 ion:
ion:
Hello,
In this case, considering the given diameter which is related to a radius of 5.0 mm and the formula for the calculation of the volume of the sphere, its volume in cubic centimeters (5.00 mm = 0.5 cm) is then:
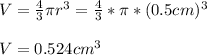
In such a way, the density turns out:
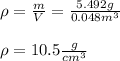
Best regards.