Answer:
Step-by-step explanation:
Given that:
Pressure P = 10 atm
number of moles of N
 = 1 mole
= 1 mole
correction for the attractive force between molecule a = 1.352
correction for the volume of molecules b = 0.0387 L mol-1
Rate = 8.314
critical Temperature Tc= 126.3 K
Using van deer Waal equation:
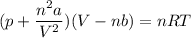
So for 1 mole of N
 gas; we have
gas; we have
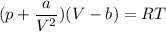
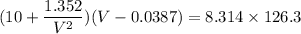
Recall that: Tc =
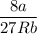
and
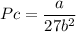
Vc = 3b
Thus;
Vc= 3×(0.0387)
Vc = 0.1161m³
using the above Van der Waal equation, the value of V can also be determined, but in several situations, we abandon the term of (V - nb) and sometimes abandon
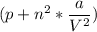 depending on the circumstances.
depending on the circumstances.