Answer:
The correct is A (A bigger mass of NaCl precipitate will form in Sample #2)
Step-by-step explanation:
From the question we are told that
The volume of each sample is
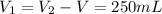
The temperature of sample one is
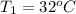
The temperature of sample two is
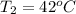
The depth from where sample two is gotten is
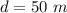
The final temperature of the samples is
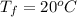
The final pressure is
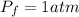
From the data given we see that the initial temperature of sample 2 is more than sample 1 so more NaCl will be dissolved in 2
Now when both samples are brought to
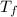 there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one
there will be a greater decrease in temperature in sample 2 than in sample 1 so more precipitate of NaCl will be formed in sample 2 than in sample one