Step-by-step explanation:
Given that,
The density of mercury is 13.5 g/mL
The density of Bromine is 3.12 g/cm³
It is mentioned that Mercury and bromine have the same mass. Let d₁,d₂ are the density of Mercury and Bromine. V₁ and V₂ are their volumes. So,
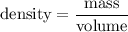
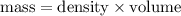
Since, mass is same.
So,
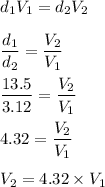
Hence, the volume of bromine is more than that of mercury. It is 4.32 times of the density of mercury.