Answer:
(a) momentum of photon is 1.205 x 10⁻²⁷ kgm/s
velocity of electron is 1323.88 m/s
momentum of the electron is 1.205 x 10⁻²⁷ kgm/s
(b) momentum of photon is 1.506 x 10⁻²⁷ kgm/s
velocity of electron is 1654.85 m/s
momentum of the electron is 1.506 x 10⁻²⁷ kgm/s
(c) The momentum of the photon is equal to the momentum of the electron
Step-by-step explanation:
(a)
wavelength of green light, λ = 550 nm
momentum of photon is given by;
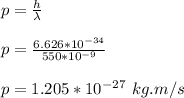
velocity of electron is given by;
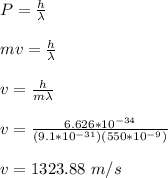
momentum of the electron is given by;
p = mv
p = (9.1 x 10⁻³¹) (1323.88)
p = 1.205 x 10⁻²⁷ kgm/s
(b)
wavelength of red light, λ = 440 nm
momentum of photon is given by;
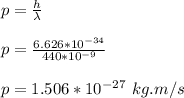
velocity of electron is given by;
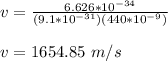
momentum of the electron is given by;
p = mv
p = (9.1 x 10⁻³¹) (1654.85)
p = 1.506 x 10⁻²⁷ kgm/s
(c) The momentum of the photon is equal to the momentum of the electron.