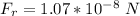Answer:
a
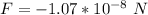
b
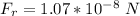
Step-by-step explanation:
Generally the force of attraction between this two irons is mathematically represented as
![F = (k * [Q_(Li) ] * [Q_(O) ] )/( r^2)](https://img.qammunity.org/2021/formulas/physics/college/4ii0qy08fwle56v1vayv3ohcy74dkwq03b.png)
Here k is known as the proportionality constant with value
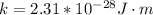
substituting -2 for
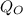 i.e the charge on oxygen , +1 for
i.e the charge on oxygen , +1 for
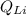 i.e the charge on Lithium and
i.e the charge on Lithium and
![[0.140 + 0.068 ] nm= 0.208 nm = 0.208*10^(-9)](https://img.qammunity.org/2021/formulas/physics/college/p9pb54ccbdpbc3vp8fbvgtd3pnukoothag.png) for r
for r
So
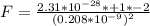
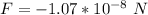
Generally the force of repulsion will be the magnitude but different direction to the force o attraction
So Force of repulsionn is