Answer:
About 512 g.
Step-by-step explanation:
We are given a sample of P₂Cl₅ that contains 179 grams of phosphorus, and we want to determine the grams of chlroine that is present.
Thus, we can convert from grams of phosphorus to moles of phosphorus, moles of phosphorus to moles of chlorine, and moles of chlorine to grams of chlorine.
From the formula, there are two moles of P for every five moles of Cl. The molecular weights of P and Cl are 30.97 g/mol and 35.45 g/mol, respectively. Hence:
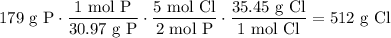
In conclusion, there is about 512 grams of chlorine present in the sample.
Alternatively, we can mass percentages. The mass percent of phosphorus in P₂Cl₅ is:
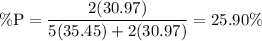
Because there are 179 grams of phosphorus, the total amount of sample present is:
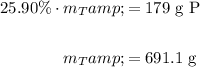
Therefore, the amount of chlorine present is 691.1 g - 179 g, or about 512 g, in agreement with our above answer.