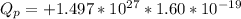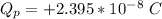Answer:
a
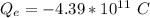
b
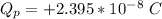
Step-by-step explanation:
Generally the number of electron in the given mass is mathematically evaluated as
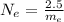
Here m_e is the mass of electron with value
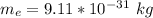
=>
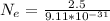
=>
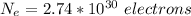
The total electric charge is mathematically represented as
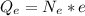
Here e is the charge on a single electron with value

So
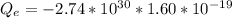
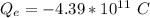
The negative sign is because we are considering electron
Generally the number of protons in the given mass is mathematically evaluated as
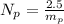
Here m_p is the mass of electron with value
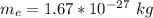
=>
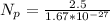
=>
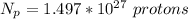
The total electric charge is mathematically represented as
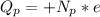
Here p is the charge on a single proton with value
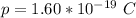
So