Answer:
![Ksp=[Zn^(2+)]^3[PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/wivcqfebt6s3whu47gb4w3urpfou4azc6j.png)
Step-by-step explanation:
Hello,
In this case, since the equilibrium dissociation of zinc phosphate is:
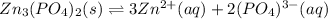
Due to the fact that only the zinc and phosphate ions are in aqueous state, the solubility product expression is written considering the law of mass action in which the concentration of each species is powered to the corresponding stoichiometric coefficient as follows:
![Ksp=[Zn^(2+)]^3[PO_4^(3-)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/wivcqfebt6s3whu47gb4w3urpfou4azc6j.png)
Best regards.