Given :
2.5 mole of Sulfuric acid
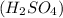 .
.
To Find :
Mass of sodium hydroxide will completely neutralize 2.5 mol of sulfuric acid
Solution :
Let us assume volume of water be 1 L .
Now , we know , to neutralize 1 mole of sulfuric acid we need 2 moles of NaOH .
So , for 2.5 mole sulfuric acid required 5 mole of NaOH .
Moles of NaOH ,
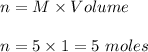
Molecular mass of NaOH , M.M = 58.44 g/mol .
Mass of 5 moles of NaOH :
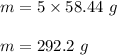
Hence , this is the required solution .