Answer:
Element symbol: Kr.
Mass number: 77.
Charge : 0.
Step-by-step explanation:
Hello,
In this case, since such substance has the same amount of protons and electrons we can infer it is an atom whose number of neutrons is defined by considering its atomic mass or mass number and atomic number which is actually equal to the number of protons and electrons (36):
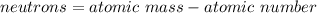
In such a way, solving for the atomic mass we obtain:
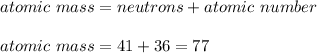
It means that the element is krypton (Kr) as it has 36 electrons and protons so its charge is 0.
Best regards.