Answer:
Mass of copper is 500.542 g.
Step-by-step explanation:
Initial temperature of copper =
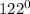 C
C
specific heat capacity of copper = 0.20 J/°C g
Mass of water = 500 g
Initial temperature of water =
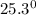 C
C
Specific heat capacity of water = 4.2 J/°C g
Final temperature of water and copper =
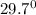 C
C
Heat loss by copper = Heat gained by water
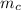
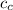 Δ
Δ
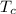 =
=
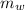
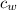 Δ
Δ
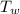
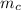 x 0.2 x (122 - 29.7) = 500 x 4.2 x (29.7 - 25.3)
x 0.2 x (122 - 29.7) = 500 x 4.2 x (29.7 - 25.3)
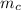 x 0.2 x 92.3 = 500 x 4.2 x 4.4
x 0.2 x 92.3 = 500 x 4.2 x 4.4
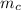 x 18.46 = 9240
x 18.46 = 9240
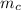 =
=
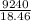
= 500.5417
mass of copper is 500.542 g.