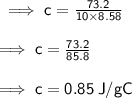Answer:

Given:
Mass (m) = 10.0 g
Rise in temperature (∆T) = 8.58°C
Energy required (Q) = 73.2 J
To Find:
Specific heat of the substance (c)
Step-by-step explanation:
Specific heat (c) of a substance is the energy required to raise the temperature of one gram of the substance by one degree Celsius.
Formula of specific heat is given as:
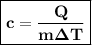
By substituting value of Q, m & ∆T in the formula we get: