Answer:
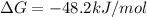
Step-by-step explanation:
Hello,
In this case, considering that the relationship between Kp and K is:
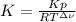
Whereas the change in the number of moles (stoichiometric coefficients) is:
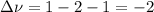
The equilibrium constant is:
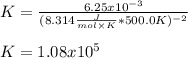
In such a way, the Gibbs free energy of reaction is:
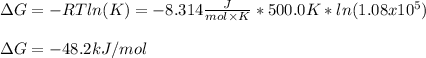
Regards.