Answer:
1) Approximately
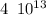 excess protons
excess protons
2) negative
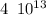 (
(
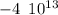 )
)
Step-by-step explanation:
1)
Recall that the charge of an electron or proton is approximately:
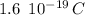
Therefore, to find the number of protons transferred in 7 micro-Coulombs of charge, we do:
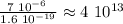
Approximately
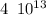 excess protons
excess protons
2)
The sign and number of uncanceled elemental charges on plate A is therefore negative
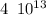 , because the same number of positive charges were removed from it, changing its neutrality
, because the same number of positive charges were removed from it, changing its neutrality