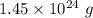Answer:
The mass of PCl₃ is 332.3 g.
The molecules of PCl₃ is
Step-by-step explanation:
Given that,
Mass of P₄ = 75.0 g
Mass of Cl₂ = 275 g
We know that,
The reaction is
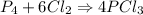
We need to calculate the moles of P₄
Using formula of moles
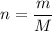
Put the value into the formula
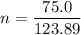
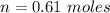
We need to calculate the moles of Cl₂
Using formula of moles
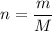
Put the value into the formula
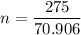
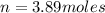
We need to calculate the number of moles of
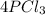
Moles in 1 P₄ = 0.61
So,
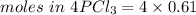
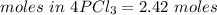
We need to calculate the mass of
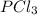
Using formula of mass
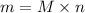
Put the value into the formula
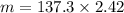
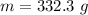
We need to calculate the molecules of
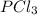
Using formula of molecules
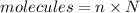
Where, n = number of moles
N = avagadro number
Put the value into the formula
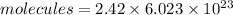
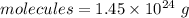
Hence, The mass of PCl₃ is 332.3 g.
The molecules of PCl₃ is