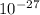In an ion with an unknown charge, the total mass of all the electrons was determined to be 1.640 x
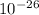
g, the total mass of its protons was 4.015 x
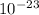
g, and the total mass of its neutrons was 4.690 x
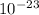
g. What is the identity, charge, and mass number of this ion? Electron one mass = 9.109 x
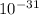
Proton one mass = 1.673 x
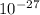
Neutron one mass = 1.675 x