Answer:
A. Substance E
A. Substance C
A. Substance A
Step-by-step explanation:
Given that:
At 4 °C, Substance E has a vapor pressure of 86. torr and Substance F has a vapor pressure of 136. torr
Which has a higher boiling point?
A. Substance E
B. Substance F
C. Neither,EandF have the same boiling point
The vapor pressure varies inversely proportional to the boiling point.
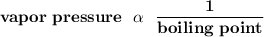
Therefore, the lower the vapor pressure, the higher the boiling point.
At 4°C, Substance E with a lower vapor pressure of 86. torr will have a higher boiling point from the given information.
2.
Recall that :
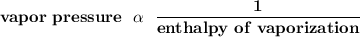
therefore, the lower the enthalpy of vaporization, the higher the vapor pressure at any given temperature.
Given that:
Substance C has an enthalpy of vaporization smaller than that of substance D. Then, substance C has a higher vapor pressure.
3.
We've earlier said that:
The vapor pressure varies inversely proportional to the boiling point.
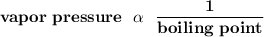
Therefore, the lower the vapor pressure, the higher the boiling point.
As such, Substance A will have a higher boiling point.