Answer:
C) A mass of 750 g and a volume of 70 dL .
Step-by-step explanation:
Hello,
In this case, for substantiating the substance having the lowest density we need to compute it in the same units for each case as shown below:
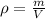
A)
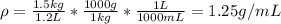
B)
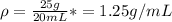
C)
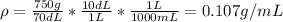
D)
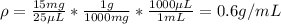
Therefore, the lowest density corresponds to C) A mass of 750 g and a volume of 70 dL
Regards.