Answer:
A )
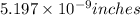
b) There are approximately 100,000,000 atoms on the 1.32 cm lines
Step-by-step explanation:
To ensure the accuracy of our answer, we will have to make sure we work in the appropriate units.
A
To convert
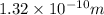 to inches, we can use this factor.
to inches, we can use this factor.
1 m = 39.3701 inches
Therefore
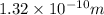 =
=
B
To find the number of atoms on a 1.32 cm line, we will first of all need to convert 1.32 cm to meters.
1.32 cm = 0.0132 metres (divided 1.32 by 100 to convert to metres)
Assuming all the atoms are arranged side by side, with their edges touching on the 0.0132m line, the number of atoms present will be
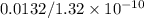 = 100,000,000 atoms
= 100,000,000 atoms