Answer:
13 grams of Oxygen
Step-by-step explanation:
Step 1: Find molecular formula of sugar
C₁₂H₂₂O₁₁
Step 2: Convert moles of oxygen present to grams
1 mol O = 16 g O
11 mol O = 176 g O
Step 3: Find molar mass of sugar
C - 12.01 g/mol
H - 1.01 g/mol
O - 16.00 g/mol
12.01(12) + 22(1.01) + 11(16.00) = 342.34 g/mol C₁₂H₂₂O₁₁
Step 4: Set up dimensional analysis
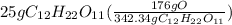
Step 5: Multiply/Divide and cancel out units
Grams of C₁₂H₂₂O₁₁ and grams of C₁₂H₂₂O₁₁ cancel out.
We are left with grams Oxygen
25/342.32 = 0.073031(176) = 12.8535 grams Oxygen
Step 6: Simplify
We only have 2 significant figures in this problem. Thus,
12.8535 = 13 g O