Answer : The metal is copper.
Explanation :
As we are given that:
Mass of metal = 25 g
Volume of metal = 40.0 mL
Formula used:
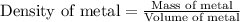
Now putting all the given values in this formula, we get:
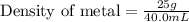
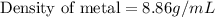
From this we conclude that the metal is copper whose density is 8.86 g/mL.
Hence, the metal is copper.