Answer:
Density = 1.74 gm/mL
Step-by-step explanation:
Given that:
Mass of piece of Magnesium = 4.80 grams
The volume displaced by this piece = 2.76mL
Let us learn about a property:
Volume of water displaced by a substance when immersed in the water is equal to the Volume of the substance itself.
So, volume of the piece of Magnesium = 2.76 mL
To find:
Density of magnesium = ?
Solution:
Let us have a look at the formula for Density of a Substance:
Density of a substance is given as the ratio of mass of substance and the volume of that mass of substance.
i.e.
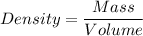
Putting the given values:
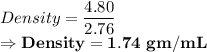
So, the answer is:
Density of Magnesium = 1.74 gm/mL