Answer:
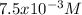
Step-by-step explanation:
Hello,
In this case, since the dissociation barium fluoride is represented at equilibrium by:
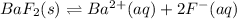
Hence, the equilibrium expression is:
![Ksp=[Ba^(2+)][F^-]^2](https://img.qammunity.org/2021/formulas/chemistry/college/3f5ozlb9rdvydxzf74frqy5hx59d25i5qy.png)
Whereas the molar solubility is represented as the reaction extent
 :
:
![Ksp=[x][2x]^2](https://img.qammunity.org/2021/formulas/chemistry/college/q20i8dezoez65zgp3iif0eoxdndn58yqlh.png)
In such a way, we can solve for
 :
:
![1.7x10^(-6)=4x^3\\\\x=\sqrt[3]{(1.7x10^(-6))/(4) } \\\\x=7.5x10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/os01hkj9n4hl20dbkocj8jr3jjg7wae4ua.png)
Which as said before, is the molar solubility.
Best regards.