Answer:
TRUE.
Step-by-step explanation:
Hello,
In this case, since the fusion enthalpy of ice is +333.9 J/g and the fusion entropy is defined as:
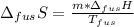
We can compute it considering the temperature (0 °C) in kelvins:
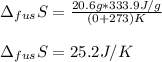
Therefore answer is TRUE.
Best regards.