Answer:
The percentage of mass of hydrogen in aspirin is 4.48%
The percentage of mass of carbon in aspirin is 60%
The percentage of mass of oxygen in aspirin is 35.5%
Step-by-step explanation:
Given that,
Mass of sample of aspirin = 134.50 g
Mass of hydrogen = 6.03 g
Mass of carbon = 80.70 g
Mass of oxygen = 47.77 g
We need to calculate the percentage of mass of hydrogen in aspirin
Using formula for percentage of mass
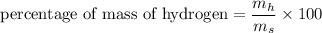
Put the value into the formula
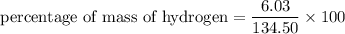
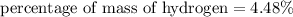
We need to calculate the percentage of mass of carbon in aspirin
Using formula for percentage of mass
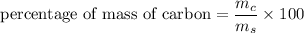
Put the value into the formula
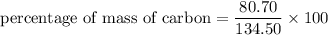
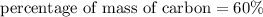
We need to calculate the percentage of mass of oxygen in aspirin
Using formula for percentage of mass
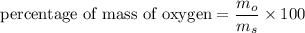
Put the value into the formula
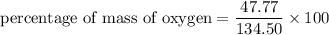
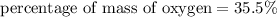
Hence, The percentage of mass of hydrogen in aspirin is 4.48%
The percentage of mass of carbon in aspirin is 60%
The percentage of mass of oxygen in aspirin is 35.5%