Answer:
0.16 M
Step-by-step explanation:
Data provided as per the question is below:-
Volume of
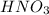 = 0.22 L
= 0.22 L
The Volume of NaOH = 0.18 L
Morality of NaOH = 0.2
According to the given situation, the calculation of the concentration of the
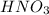 is shown below:-
is shown below:-
For equivalence,
Number of the equivalent of
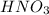 = Number of equivalents of NaOH
= Number of equivalents of NaOH
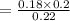
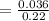
= 0.16363 M
or
= 0.16 M