Answer:
0.5 g/L.
Step-by-step explanation:
Hello,
In this case, for this solubility problem, we can write for the lead (II) fluoride:
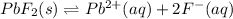
And the equilibrium expression is:
![Ksp=[Pb^(2+)][F^-]^2](https://img.qammunity.org/2021/formulas/chemistry/college/nc5bo2lp5dbb9oq5um8g2urwm8a14iwo4k.png)
Whereas Ksp of lead (II) fluoride is 3.3x10⁻⁸. In such a way, we can write the equilibrium expression in terms of the molar solubility
 as follows:
as follows:
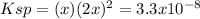
Hence, solving for
 we find:
we find:
![x=\sqrt[3]{(3.3x10^(-8))/(4) }\\\\x=2.02x10^(-3)M](https://img.qammunity.org/2021/formulas/chemistry/college/7nqvafvor0e6de6yrwh2dfg5gzma7i72g1.png)
Moreover, since the molar mass of lead (II) fluoride is 245.2 g/mol, the solubility turns out:
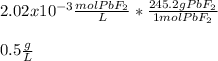
Best regards.