Answer:
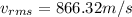
Step-by-step explanation:
Hello,
In this case, since the rms speed of the molecules is computed by:
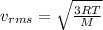
Whereas the absolute temperature is computed from the internal energy (by using the Cp of helium (3.1156 J/g*K) as shown below:
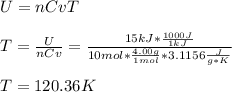
Thereby, the rms speed results:
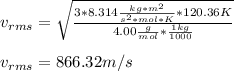
Regards.