Answer:
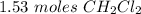 .
.
Step-by-step explanation:
We can start with the reaction, if we know the formula for each compound:
-) Methane:
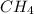
-) Carbon tetrachloride:
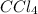
-) Dichloromethane:
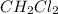
With this in mind, we can write the reaction:
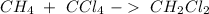
Now, we can balance the reaction:
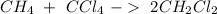
After this, we have 2 carbon atoms on each side, 4 hydrogen atom on each side, and 4 chlorine atoms on each side.
If we want to know how many moles of
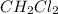 would be produced with .766 moles of
would be produced with .766 moles of
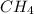 , we have to check the balanced reaction and use the molar ratio. In this case, the molar ratio is 1 mol
, we have to check the balanced reaction and use the molar ratio. In this case, the molar ratio is 1 mol
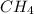 will produce 2 moles of
will produce 2 moles of
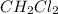 (1:2). So:
(1:2). So:
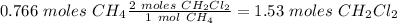
We wil have
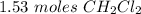 .
.
I hope it helps!