Answer:
e. Lead
Step-by-step explanation:
Hello,
In this case, since the equation to compute the heat in a heating or a cooling process is:
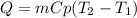
We can see that the lower the specific heat of the substance, the higher the reached temperature as they are in an inversely proportional relationship. In such a way, we can say that e. Lead will reach the higher temperature if the same heat is added to same mass of the other metals.
Regards.