Answer:
The percentage loss in mass between sucrose solutions 0.5 mol/dm³ and 0.1 mol/dm³ is 80.0% loss in mass
Step-by-step explanation:
The molecular formula of sucrose is C₁₂H₂₂O₁₁
The molecular mass of sucrose is 342.3 g/mol
Therefore;
The mass of sucrose in 0.1 mol/dm³ solution = 0.1 × 342.3 = 34.23 g
The mass of sucrose in 0.5 mol/dm³ solution = 0.5 × 342.3 = 171.5 g
The percentage loss in mass of the sucrose is given as follows;
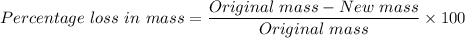
Therefore;
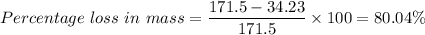
Which gives, the percentage loss in mass between sucrose solutions 0.1 mol/dm³ and 0.5 mol/dm³ is 80.0% to three significant figures.