The given question is incomplete. The complete question is :
For the reaction
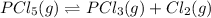 at equilibrium, which statement correctly describes the effects of increasing pressure and adding
at equilibrium, which statement correctly describes the effects of increasing pressure and adding
 , respectively
, respectively
a) Increasing pressure causes shift to reactants, adding
 causes shift to products.
causes shift to products.
b) Increasing pressure causes shift to products ,adding
 causes shift to reactants.
causes shift to reactants.
c) Increasing pressure causes shift to products, adding
 causes shift to products.
causes shift to products.
d) Increasing pressure causes shift to reactants,adding
 causes shift to reactants
causes shift to reactants
Answer: Increasing pressure causes shift to reactants, adding
 causes shift to products.
causes shift to products.
Step-by-step explanation:
Any change in the equilibrium is studied on the basis of Le-Chatelier's principle.
This principle states that if there is any change in the variables of the reaction, the equilibrium will shift in the direction to minimize the effect.
For the given equation:
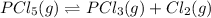
a) If the pressure is increased, the volume will decrease according to Boyle's Law. Now, according to the Le-Chatlier's principle, the equilibrium will shift in the direction where decrease in pressure is taking place. As the number of moles of gas molecules is lesser at the reactant side. So, the equilibrium will shift in the left direction. i.e. towards reactants.
b) If
 is added, the equilibrium will shift in the direction where
is added, the equilibrium will shift in the direction where
 is decreasing. So, the equilibrium will shift in the right direction. i.e. towards products.
is decreasing. So, the equilibrium will shift in the right direction. i.e. towards products.