Answer:
The percentage error is -2.4 %
Step-by-step explanation:
The percentage error is calculated by the following formula;
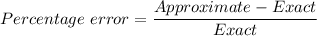
The given parameters are;
The atomic mass of cobalt = 58.933 amu. = The exact value
The mass of cobalt as measured by the scientist = 57.514 = The approximate value
Therefore, the percentage error is given plugging in the values into the above equation for percentage error as follows;
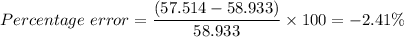
The percentage error = -2.4 %.