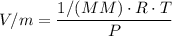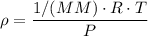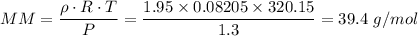Answer:
The molar mass = 39.4 g/mol
Step-by-step explanation:
The given parameters are;
The pressure at which the gas was measured = 1.3 atm.
The temperature of the gas = 47°C
The measured density of the gas = 1.95 g/L
By the combined gas equation, we have;
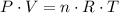
Where;
n = Number of moles = m/MM
P = Pressure = 1.3 atm
V = Specific, Molar Volume
T = Temperature = 47 °C = 320.15 K
R = Universal Gas Constant = 0.08205 L·atm/(mol·K)
ρ = The density = MM/V
Where;
MM = Molar mass
Therefore, V = MM×ρ